Health officials warn consumers about fake Botox
SACRAMENTO – Counterfeit versions of Botox (botulinum toxin) have been found in multiple states, including California, according to an alert issued recently by the California Department of Public Health (CDPH).
These fake products have resulted in hospitalizations and other serious reactions in people who received injections in non-medical, unlicensed settings.
“Counterfeit or incorrectly administered Botox, even in small amounts, can result in serious health problems and even death,” said CDPH Director and State Public Health Officer Dr. Tomás J. Aragón.
“Consumers should only get injections of FDA-approved Botox from licensed and trained professionals in healthcare settings. Botox should never be purchased online or through unlicensed individuals.”
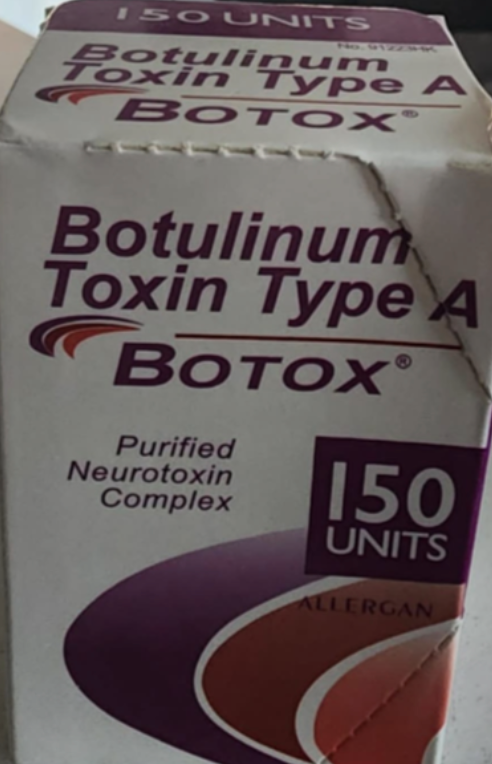
One of the examples of counterfeit botox packaging. Image: California Department of Public Health website
CDPH urges those who have received or are considering Botox injection for medical or cosmetic purposes to take these precautions:
- Confirm with your healthcare professional that you are receiving Botox from an authorized source.
- Ask your healthcare professional if they are licensed and trained to administer Botox.
- If in doubt, do not get the injection.
- Contact a healthcare professional or go to the emergency room immediately if you have any symptoms of botulism poisoning.
In California Botox treatments may be performed by a physician or by a registered nurse or physician assistant under a physician’s supervision.
More information on cosmetic procedures can be found on the Medical Board of California’s website.
Botulism poisoning
Symptoms caused by counterfeit Botox are similar to botulism poisoning and include drooping eyelids, difficulty swallowing, dry mouth, slurred speech, difficulty breathing, fatigue, and generalized weakness.
Consumers should report suspected counterfeit Botox products through the FDA’s website or by calling 800-551-3989. Suspected counterfeit Botox products in California can be reported to CDPH at Consumer Complaints.
Healthcare professionals and consumers should report adverse events related to the use of any medications, including suspected counterfeit medications, to the FDA’s MedWatch Safety Information and Adverse Event Reporting Program.
Information for healthcare professionals
Botulism is considered a medical and public health emergency. If you suspect botulism in a patient, contact your local health department immediately and consider the following:
- Report to your local health department any adverse events following the administration of Botox.
- Check the product for any signs of counterfeiting before administering.
- Federal law requires that all healthcare providers who dispense or administer prescription drugs purchase products only from authorized sources. Purchasing and administering counterfeit products is illegal and puts patients at risk.
- Visit the FDA’s website for information about how to safely purchase prescription drugs for your patients.
- Know Your Source: Protecting Patients from Unsafe Drugs.

Counterfeit versions of Botox. Image: California Department of Public Health website
Signs of counterfeit versions of Botox
The counterfeit product may be identified by one or more of the following:
Outer carton/ packaging
- Displays the active ingredient as “Botulinum Toxin Type A” instead of “OnabotulinumtoxinA”
- Indicates 150-unit doses
- Contain lot number C3709C3
- Includes language that is not English
The vial
- Indicates 150-unit doses
- Contain lot number C3709C3

